Abstract
Introduction: IMGN779 is a next-generation anti-CD33 ADC with a novel DNA-alkylating IGN payload and a cleavable s-SPDB linker. Preclinical studies suggest potent antitumor activity and improved safety and tolerability compared with DNA-crosslinking payloads, allowing for repeat dosing. Here we report the initial safety and antileukemia activity from the dose escalation phase of the IMGN779 first-in-human trial.
Objectives: To determine the dose-limiting toxicity (DLT) and maximum tolerated dose (MTD), safety, pharmacokinetics (PK), pharmacodynamics (PD), and preliminary antileukemia activity of IMGN779.
Methods: Informed consent was obtained from eligible adult patients (≥ 18 years) with relapsed or refractory CD33+ acute myeloid leukemia (AML) (i.e., ≥20% of blasts expressing CD33). IMGN779 was administered intravenously on Days 1 and 15 of a 28-day cycle in a standard 3+3 design.
Results: Twenty-six patients with a median age of 67 years (range: 34-81) have received IMGN779. Twenty-one (81%) had primary refractory or >1st relapse AML, and 17 (65%) had received intense frontline therapy, including stem cell transplant in 5 (19%) patients.
Safety : No DLT has been observed at doses up to 0.7 mg/kg (cohort 8). The most common Grade 3+ adverse events (AEs) were febrile neutropenia (39%), pneumonia (19%), anemia (19%), respiratory failure (15%), and hypophosphatemia (12%). Other Grade 3+ AEs of clinical interest included 1 Grade 3 infusion reaction (4%) (nonrecurrent on rechallenge), 1 Grade 3 alanine aminotransferase elevation (4%), and 2 Grade 3 bilirubin elevations (8%) that did not meet criteria for drug-induced liver injury. With a median of 3 doses (range: 1-20) administered per patient, there has been no evidence of cumulative toxicity. There has been no correlation between increasing dose and the frequency, nature, or severity of the AEs observed. There have been no study drug-related deaths.
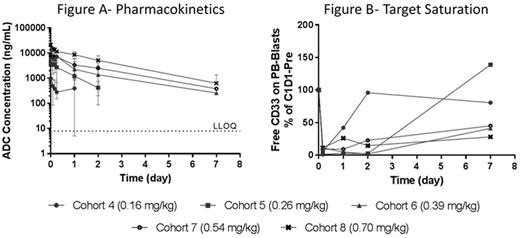
PK/PD: There are dose-dependent increases in Cmax and AUC with prolonged exposure (Figure A) and increased duration (extending to 7 days in cohorts 6-8, 0.39-0.7 mg/kg) of target saturation at higher doses (Figure B). The data appear to be consistent with target-mediated drug disposition (TMDD), where variability in PK is attributable to dose level and intrapatient differences in target abundance, with dose-proportional exposures observed at higher dose levels (cohorts 5-8, 0.26-0.7 mg/kg).
Antileukemia activity: In cohorts 6-8 (0.39-0.7 mg/kg), all 9 patients showed decreased peripheral blasts within 3-8 days of first dose, with a median maximal reduction of 67% (range: 15%-100%). Four patients whose peripheral blood blasts increased again before Day 15 showed a subsequent decrease in blasts after the second dose (Day 15). Importantly, 3 patients in these later cohorts showed a substantial decrease in bone marrow blasts within the first 3 cycles (without hydroxyurea): 90% to 4% (96% decrease) met criteria for morphologic leukemia-free state but died in Cycle 2 due to unrelated AE; 54% to 5% (90% decrease) and continued into Cycle 4; 23% to 12% (48% decrease) but progressed in Cycle 2.
Conclusions: These preliminary findings demonstrate initial antileukemia activity with IMGN779, a next-generation anti-CD33 ADC, in relapsed or refractory adult AML, with a toxicity profile consisting of isolated events most consistent with underlying disease and/or comorbidity. In the context of limited patient numbers, antileukemia activity was seen in patients with ADC exposure of less than 48 hours. IMGN779 PD shows consistent prolongation of CD33 saturation with increasing dose level and rebound of CD33 antigen with loss of detectable ADC. In addition, repeat dosing leads to deepening blood and bone marrow responses. Alignment between PK/PD and antileukemia activity is emerging as dose escalation and schedule optimization continue.
Cortes: Teva: Research Funding; BMS: Consultancy, Research Funding; ARIAD: Consultancy, Research Funding; Novartis Pharmaceuticals Corporation: Consultancy, Research Funding; Pfizer: Consultancy, Research Funding; Sun Pharma: Research Funding; ImmunoGen: Consultancy, Research Funding. Traer: ImmunoGen: Consultancy; Tolero: Consultancy; Notable Labs: Equity Ownership. Erba: Celgene: Consultancy, Other: Chair, Scientific Steering Committee , Speakers Bureau; Incyte: all research support paid to University of Alabama, Consultancy, Speakers Bureau; Jazz: Consultancy, Speakers Bureau; Novartis: Consultancy, Speakers Bureau; Amgen: Consultancy, Other: all research support paid to University of Alabama, Research Funding; Daiichi Sankyo: Consultancy, Other: all research support paid to University of Alabama, Research Funding; ImmunoGen: Consultancy, Other: all research support paid to University of Alabama, Research Funding; MacroGen: Consultancy; Ono: Consultancy; Pfizer: Consultancy; Seattle Genetics: Consultancy, Other: all research support paid to University of Alabama, Research Funding; Sunesis: Consultancy; Millennium/Takeda: Consultancy, Other: all research support paid to University of Alabama, Research Funding; Agios: Other: all research support paid to University of Alabama, Research Funding; Juno: Other: all research support paid to University of Alabama, Research Funding; Astellas: Other: all research support paid to University of Alabama, Research Funding; Celator: Other: all research support paid to University of Alabama, Research Funding; Janssen: Other: all research support paid to University of Alabama, Research Funding; Glycomimetics: Other: Chair, Data and Safety Monitoring Committee. Blum: Astellas: Consultancy; Boerhinger Ingelheim: Research Funding; Pfizer: Consultancy. Sloss: ImmunoGen: Employment. Culm-Merdek: ImmunoGen: Employment. Towles: ImmunoGen: Consultancy. Zweidler-McKay: ImmunoGen: Employment. DeAngelo: Celgene: Research Funding; Immunogen: Honoraria, Research Funding; Blueprint Medicines: Honoraria, Research Funding; Glycomimetics: Research Funding; Novartis Pharmaceuticals Corporation: Consultancy, Honoraria, Research Funding; Pfizer Inc.: Consultancy, Honoraria, Research Funding; BMS: Consultancy; Shire: Honoraria; Amgen: Consultancy, Research Funding; ARIAD: Consultancy, Research Funding; Takeda Pharmaceuticals U.S.A., Inc.: Honoraria; Incyte: Consultancy, Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal